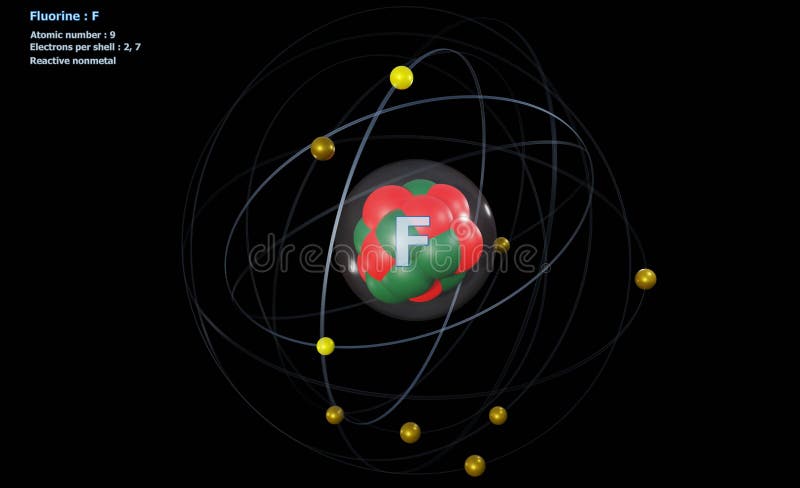
Name: Fluorine: Symbol: F: Atomic Number: 9: Atomic Mass: 18.998 atomic mass units: Number of Protons: 9: Number of Neutrons: 10: Number of Electrons: 9: Melting. Fluorine has seven valence electrons. Total number of valence electrons for SF 2 – 6 + 7.2 ( as there are two atoms of Fluorine, we will multiply the number by 2) = 6 + 14 = 20 valence electrons. So, Sulphur Difluoride has a total of 20 valence electrons. SF2 Lewis Structure. Lewis Structure is the pictorial representation of the arrangement. Fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. The chemical symbol for Fluorine is F.
Fluorine (F) is the first element in the Halogen group (group 17) in the periodic table. Its atomic number is 9 and its atomic weight is 19, and it's a gas at room temperature. It is the most electronegative element, given that it is the top element in the Halogen Group, and therefore is very reactive. It is a nonmetal, and is one of the few elements that can form diatomic molecules (F2). It has 5 valence electrons in the 2p level. Its electron configuration is 1s22s22p5. It will usually form the anion F- since it is extremely electronegative and a strong oxidizing agent. Fluorine is a Lewis acid in weak acid, which means that it accepts electrons when reacting. Fluorine has many isotopes, but the only stable one found in nature is F-19.
Quick Reference Table
- Each subshell is constrained to hold 4ℓ + 2 electrons at most, namely: Each s subshell holds at most 2 electrons Each p subshell holds at most 6 electrons Each d subshell holds at most 10 electrons.
- Fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. The chemical symbol for Fluorine is F. Fluorine is the lightest halogen and exists as a highly toxic pale yellow diatomic gas at standard conditions.
| Symbol | F |
|---|---|
| Atomic Number | 9 |
| Group | 17 (Halogens) |
| Electron Configuration | 1s22s22p5 |
| Atomic Weight | 18.998 g |
| Density | 1.7 g/L |
| Melting Point | -219.62oC |
| Boiling Point | -188.12oC |
| Critical Point | 144.13K, 5.172 MPa |
| Oxidation States | -1 |
| Electronegativity | 3.98 |
| Stable Isotopes | F-19 |
Brief History
In the late 1600's minerals which we now know contain fluorine were used in etching glass. The discovery of the element was prompted by the search for the chemical substance which was able to attack glass (it is HF, a weak acid). The early history of the isolation and work with fluorine and hydrogen fluoride is filled with accidents since both are extremely dangerous. Eventually, electrolysis of a mixture of KF and HF (carefully ensuring that the resulting hydrogen and fluorine would not come in contact) in a platinum apparatus yielded the element.
Fluorine was discovered in 1530 by Georgius Agricola. He originally found it in the compound Fluorspar, which was used to promote the fusion of metals. It was under this application until 1670, when Schwanhard discovered its usefulness in etching glass. Pure fluorine (from the Latin fluere, for 'flow') was was not isolated until 1886 by Henri Moissan, burning and even killing many scientists along the way. It has many uses today, a particular one being used in the Manhattan project to help create the first nuclear bomb.
Electronegativity of Fluorine
Fluorine is the most electronegative element on the periodic table, which means that it is a very strong oxidizing agent and accepts other elements' electrons. Money controls driver download for windows 10. Fluorine's atomic electron configuration is 1s22s22p5. (see Figure 2)
Fluorine is the most electronegative element because it has 5 electrons in it's 2P shell. The optimal electron configuration of the 2P orbital contains 6 electrons, so since Fluorine is so close to ideal electron configuration, the electrons are held very tightly to the nucleus. The high electronegativity of fluorine explains its small radius because the positive protons have a very strong attraction to the negative electrons, holding them closer to the nucleus than the bigger and less electronegative elements.
Reactions of Fluorine
Because of its reactivity, elemental fluorine is never found in nature and no other chemical element can displace fluorine from its compounds. Fluorine bonds with almost any element, both metals and nonmetals, because it is a very strong oxidizing agent. It is very unstable and reactive since it is so close to its ideal electron configuration. It forms covalent bonds with nonmetals, and since it is the most electronegative element, is always going to be the element that is reduced. It can also form a diatomic element with itself ((F_2)), or covalent bonds where it oxidizes other halogens ((ClF), (ClF_3), (ClF_5)). It will react explosively with many elements and compounds such as Hydrogen and water. Elemental Fluorine is slightly basic, which means that when it reacts with water it forms (OH^-).
[3F_2+2H_2O rightarrow O_2+4HF tag{1}]
When combined with Hydrogen, Fluorine forms Hydrofluoric acid ((HF)), which is a weak acid. This acid is very dangerous and when dissociated can cause severe damage to the body because while it may not be painful initially, it passes through tissues quickly and can cause deep burns that interfere with nerve function.

[HF+H_2O rightarrow H_3O^++F^- tag{2}]
There are also some organic compounds made of Fluorine, ranging from nontoxic to highly toxic. Fluorine forms covalent bonds with Carbon, which sometimes form into stable aromatic rings. When Carbon reacts with Fluorine the reaction is complex and forms a mixture of (CF_4), (C_2F_6), an (C_5F_{12}).
[C_{(s)} + F_{2(g)} rightarrow CF_{4(g)} + C_2F_6 + C_5F_{12} tag{3}]
Fluorine reacts with Oxygen to form (OF_2) because Fluorine is more electronegative than Oxygen. The reaction goes:
[2F_2 + O_2 rightarrow 2OF_2 tag{4}]
Fluorine is so electronegative that sometimes it will even form molecules with noble gases like Xenon, such as the the molecule Xenon Difluoride, (XeF_2).
[Xe + F_2 rightarrow XeF_2 tag{5}]
Fluorine also forms strong ionic compounds with metals. Some common ionic reactions of Fluorine are:
[F_2 + 2NaOH rightarrow O_2 + 2NaF +H_2 tag{6}]


[4F_2 + HCl + H_2O rightarrow 3HF + OF_2 + ClF_3 tag{7}]
[F_2 + 2HNO_3 rightarrow 2NO_3F + H_2 tag{8}]
Applications of Fluorine
Compounds of fluorine are present in fluoridated toothpaste and in many municipal water systems where they help to prevent tooth decay. And, of course, fluorocarbons such as Teflon have made a major impact on life in the 20th century. There are many applications of fluorine:
- Rocket fuels
- Polymer and plastics production
- teflon and tefzel production
- When combined with Oxygen, used as a refrigerator cooler
- Hydrofluoric acid used for glass etching
- Purify public water supplies
- Uranium production
- Air conditioning
Sources
Fluorine can either be found in nature or produced in a lab. To make it in a lab, compounds like Potassium Fluoride are put through electrolysis with Hydrofluoric acid to create pure Fluorine and other compounds. It can be carried out with a variety of compounds, usually ionic ones involving Fluorine and a metal. Fluorine can also be found in nature in various minerals and compounds. The two main compounds it can be found in are Fluorspar ((CaF_2)) and Cryolite ((Na_3AlF_6)).
References
- Newth, G. S. Inorganic Chemistry. Longmans, Green, and Co.:New York, 1903.
- Latimer, Wendell M., Hildebrand, Joel H. Reference Book of Inorganic Chemistry. The Macmillan Company: New York, 1938.
Problems
(Highlight to view answers)
1. Q. What is the electron configuration of Fluorine? of F-?
A. 1s22s22p5
1s22s22p6
2. Q. Is Fluorine usually oxidized or reduced? explain.
A. Fluorine is usually reduced because it accepts an electron from other elements since it is so electronegative.
3. Q. What are some common uses of Fluorine?
A. Toothpaste, plastics, rocket fuels, glass etching, etc.
4. Q. Does Fluorine form compounds with nonmetals? if so, give two examples, one of them being of an oxide.
A. OF2, ClF
5. Q. What group is Fluorine in? (include name of group and number)
Number Of Electrons In Boron
A. 17, Halogens
Contributors and Attributions
- Rachel Feldman (University of California, Davis)
Stephen R. Marsden
Our editors will review what you’ve submitted and determine whether to revise the article.
Join Britannica's Publishing Partner Program and our community of experts to gain a global audience for your work!Fluorine (F), most reactive chemical element and the lightest member of the halogen elements, or Group 17 (Group VIIa) of the periodic table. Its chemical activity can be attributed to its extreme ability to attract electrons (it is the most electronegative element) and to the small size of its atoms.
| atomic number | 9 |
|---|---|
| atomic weight | 18.998403163 |
| melting point | −219.62 °C (−363.32 °F) |
| boiling point | −188 °C (−306 °F) |
| density (1 atm, 0 °C or 32 °F) | 1.696 g/litre (0.226 ounce/gallon) |
| oxidation states | −1 |
| electron config. | 1s22s22p5 |
History
The fluorine-containing mineral fluorspar (or fluorite) was described in 1529 by the German physician and mineralogist Georgius Agricola. It appears likely that crude hydrofluoric acid was first prepared by an unknown English glassworker in 1720. In 1771 the Swedish chemist Carl Wilhelm Scheele obtained hydrofluoric acid in an impure state by heating fluorspar with concentrated sulfuric acid in a glass retort, which was greatly corroded by the product; as a result, vessels made of metal were used in subsequent experiments with the substance. The nearly anhydrous acid was prepared in 1809, and two years later the French physicist André-Marie Ampère suggested that it was a compound of hydrogen with an unknown element, analogous to chlorine, for which he suggested the name fluorine. Fluorspar was then recognized to be calcium fluoride.
The isolation of fluorine was for a long time one of the chief unsolved problems in inorganic chemistry, and it was not until 1886 that the French chemist Henri Moissan prepared the element by electrolyzing a solution of potassium hydrogen fluoride in hydrogen fluoride. He received the 1906 Nobel Prize for Chemistry for isolating fluorine. The difficulty in handling the element and its toxic properties contributed to the slow progress in fluorine chemistry. Download legend usb devices driver. Indeed, up to the time of World War II the element appeared to be a laboratory curiosity. Then, however, the use of uranium hexafluoride in the separation of uranium isotopes, along with the development of organic fluorine compounds of industrial importance, made fluorine an industrial chemical of considerable use. Msc vertriebs driver download.
Occurrence and distribution
The fluorine-containing mineral fluorspar (fluorite, CaF2) has been used for centuries as a flux (cleansing agent) in various metallurgical processes. The name fluorspar is derived from the Latin fluere, “to flow.” The mineral subsequently proved to be a source of the element, which was accordingly named fluorine. The colourless, transparent crystals of fluorspar exhibit a bluish tinge when illuminated, and this property is accordingly known as fluorescence.
Number Of Protons And Electrons For Fluorine
Fluorine is found in nature only in the form of its chemical compounds, except for trace amounts of the free element in fluorspar that has been subjected to radiation from radium. Not a rare element, it makes up about 0.065 percent of Earth’s crust. The principal fluorine-containing minerals are (1) fluorspar, deposits of which occur in Illinois, Kentucky, Derbyshire, southern Germany, the south of France, and Russia and the chief source of fluorine, (2) cryolite (Na3AlF6), chiefly from Greenland, (3) fluoroapatite (Ca5[PO4]3[F,Cl]), widely distributed and containing variable amounts of fluorine and chlorine, (4) topaz (Al2SiO4[F,OH]2), the gemstone, and (5) lepidolite, a mica as well as a component of animal bones and teeth.
Physical and chemical properties
At room temperature fluorine is a faintly yellow gas with an irritating odour. Inhalation of the gas is dangerous. Upon cooling fluorine becomes a yellow liquid. There is only one stable isotope of the element, fluorine-19.
Because fluorine is the most electronegative of the elements, atomic groupings rich in fluorine are often negatively charged. Methyl iodide (CH3I) and trifluoroiodomethane (CF3I) have different charge distributions as shown in the following formulas, in which the Greek symbol δ indicates a partial charge:
The first ionization energy of fluorine is very high (402 kilocalories per mole), giving a standard heat formation for the F+ cation of 420 kilocalories per mole.
The small size of the fluorine atom makes it possible to pack a relatively large number of fluorine atoms or ions around a given coordination centre (central atom) where it forms many stable complexes—for example, hexafluorosilicate (SiF6)2− and hexafluoroaluminate (AlF6)3−. Fluorine is the most powerfully oxidizing element. No other substance, therefore, is able to oxidize the fluoride anion to the free element, and for this reason the element is not found in the free state in nature. For more than 150 years, all chemical methods had failed to produce the element, success having been achieved only by the use of electrolytic methods. However, in 1986 American chemist Karl O. Christe reported the first chemical preparation of fluorine, where “chemical preparation” means a method that does not use techniques such as electrolysis, photolysis, and discharge or use fluorine itself in the synthesis of any of the starting materials. He used K2MnF6 and antimony pentafluoride (SbF5), both of which can be easily prepared from HF solutions.
The high oxidizing power of fluorine allows the element to produce the highest oxidation numbers possible in other elements, and many high oxidation state fluorides of elements are known for which there are no other corresponding halides—e.g., silver difluoride (AgF2), cobalt trifluoride (CoF3), rhenium heptafluoride (ReF7), bromine pentafluoride (BrF5), and iodine heptafluoride (IF7).
Fluorine Numbers Valence Electron
Fluorine (F2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. Some metals, such as nickel, are quickly covered by a fluoride layer, which prevents further attack of the metal by the element. Certain dry metals, such as mild steel, copper, aluminum, or Monel (a 66 percent nickel, 31.5 percent copper alloy), are not attacked by fluorine at ordinary temperatures. For work with fluorine at temperatures up to 600 °C (1,100 °F), Monel is suitable; sintered alumina is resistant up to 700 °C (1,300 °F). When lubricants are required, fluorocarbon oils are most suitable. Fluorine reacts violently with organic matter (such as rubber, wood, and cloth), and controlled fluorination of organic compounds by the action of elemental fluorine is only possible if special precautions are taken.
Number Of Electrons In Fluorine Ion
Element Fluorine Protons Neutrons Electrons
- key people
- related topics
